Electrolysis is a process that removes rust in a piece of metal. It works by placing two metals in water and applying voltage. But what type of battery charger is best for electrolysis? After our thorough research, we've discovered the answer.
Electrolysis uses a DC power supply, so it's a good idea to use a battery charger specifically designed for DC power. Most car battery chargers fit the bill, so you can use your existing car battery charger to get started.
The concept of electrolytic cleaning is based on placing the metals (anode and cathode) into an electrolytic solution. One metal takes the beating while the other is de-rusted. We'll show you how to make a DIY electrolytic cleaner that removes rust from metal items without harsh chemicals, so keep reading!
How Does Electrolytic Cleaning Work
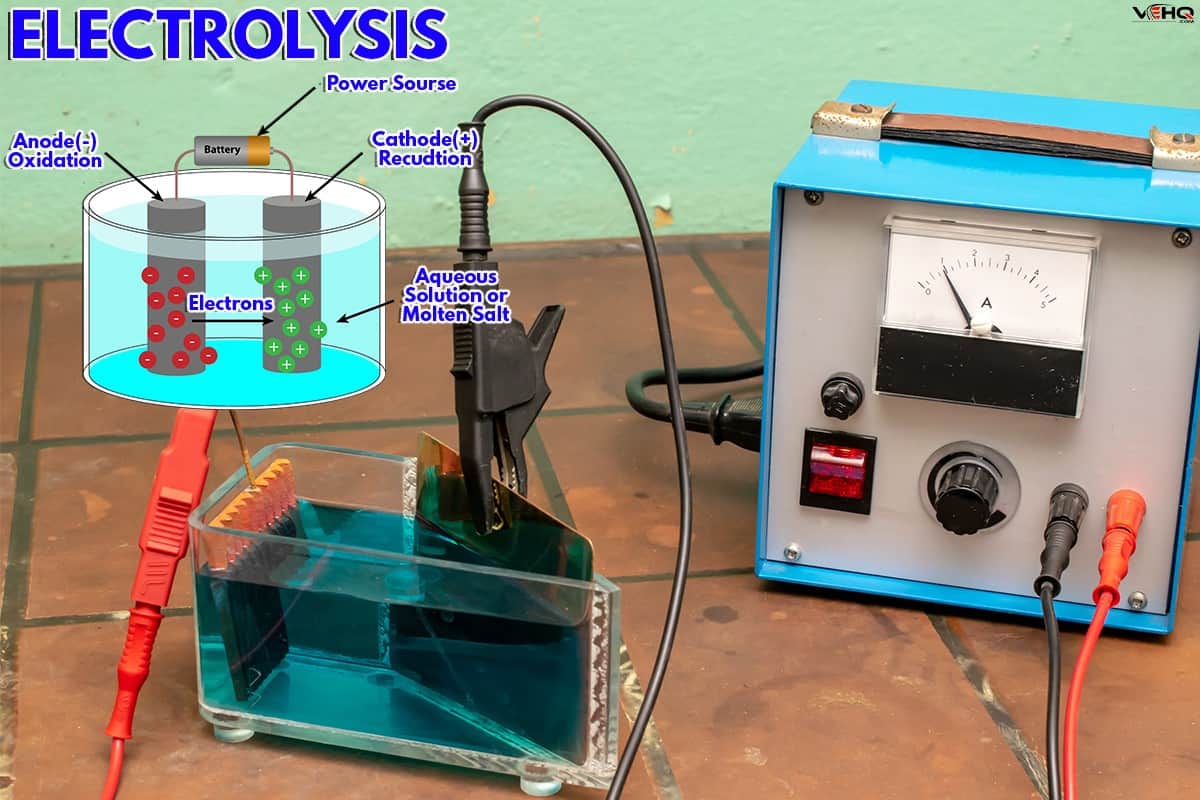
Electrolysis is the method of using electricity to create an electrolytic reaction to clean metal parts and objects. It is a better alternative to scouring pads and detergents because you can dissolve even the most stubborn rust in an electrolytic reaction with less effort.
An electrolytic reaction is the process of dissolving rust by applying electricity to the object. When a piece of metal is immersed in an electrolysis bath, an electric current will flow through the electrolytic solution to the metal.
This causes the ionization of the water in the electrolytic solution, which causes the rust to come off and migrate to the sacrificial metal. As they move to the surface of the sacrificial metal, they create bubbles that rise to the surface.
How To Make Your DIY Electrolytic Cleaner
Materials needed:
- A container with water
- Some sacrificial metals.
- Washing soda
- A car battery.
- A car battery charger.
- Booster cables
Here are the steps to follow:
1. Pour some water into the container.
You'll be submerging the metal part or object that needs de-rusting so you'll need a container that is big enough to accommodate it. Add a cup of washing soda.
2. Place the sacrificial metals (anodes) into the container.

These metals will be charged and will take a beating as they attract rust from the metal or object being de-rusted. Make sure you don't need for them anymore.
3. Hook the car battery charger connectors to the battery terminal.
As a general rule of thumb, red goes to the positive terminal and black goes to the negative terminal. You can use a dead battery since you'll be drawing voltage from the car battery charger.
However, if you're planning to use a dead battery, then you need a simple car battery charger and not a smart battery charger.
Since a smart battery charger is has more complicated tech, it might think there's something wrong with the battery, causing it to shut down instead of continuously charging the battery.
Check out this manual car battery charger on Amazon.
4. Hook the booster cable
Using the same color coding, connect the booster cable to the battery terminal as well.
Check out these booster cables on Amazon.
5. Attach the gator clips to the metal
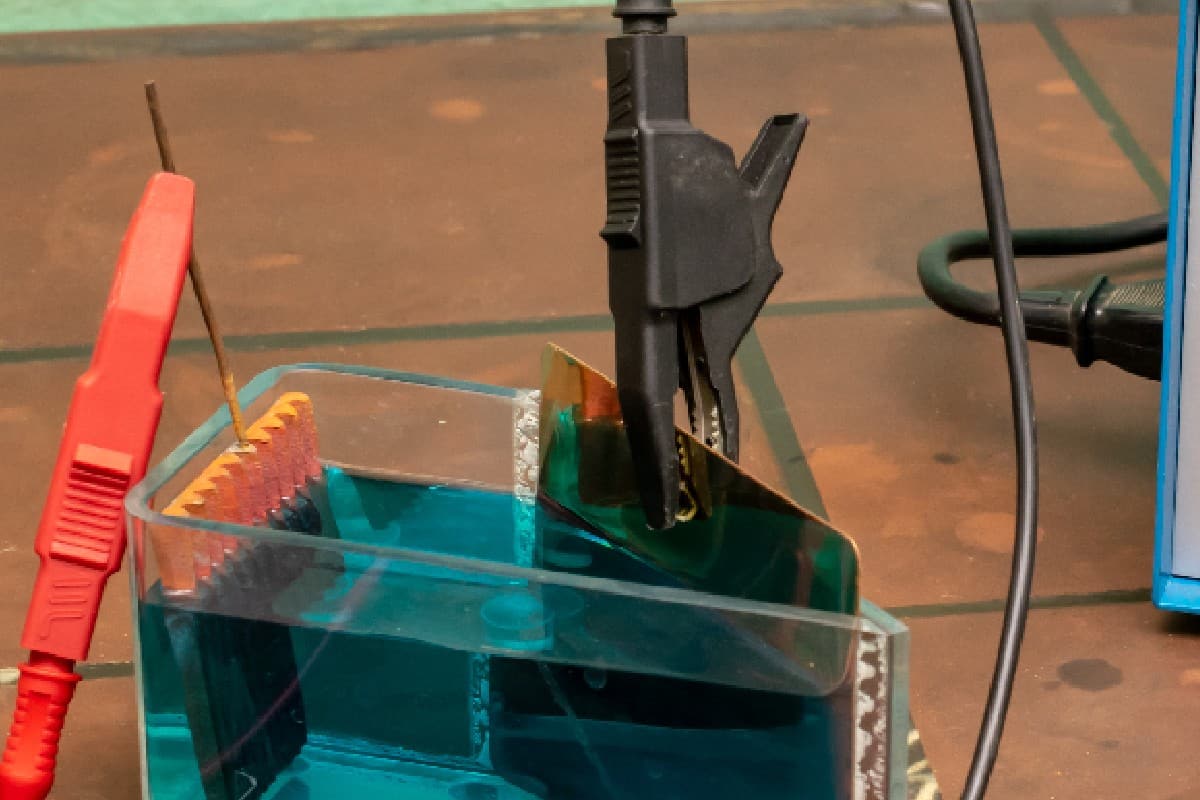
Attach the positive gator clip to the sacrificial metals and the negative gator clip to the object you're de-rusting.
6. Let it sit for a few hours
Let the electrolysis do its job. For moderate cases of rust, 4 to 6 hours should be enough to see results. For severe cases, it can take up to 12 hours.
You'll observe that the sacrificial metals will develop rust over time. Don't worry. That's how they're supposed to behave, and it's a sign that the electrolysis is working.
Do Amps Matter for Electrolytic Cleaning?
Yes, it does. Amperage matters a great deal in an electrolysis setup, as it needs to be considered in order to ensure the right electrolytic conditions. A high amperage level increases the current density within the solution. This means that as the amperage used to electrolyze the solution increases, the rate of dissolution is expediated.
How Many Amps for Electrolytic Cleaning?

The typical current capacity of an electrolysis bath should be 10 amps. This enables the electrolytic solution to dissolve rust at a much faster rate than if you had an electrolytic solution operating at lower amperage.
However, it’s not likely that you can deliver a constant flow of 10 amps unless you have an anode that covers a wide surface area that's enough to support the flow.
If your anode is too narrow in width, then the current will be concentrated on a small area, and will therefore not dissolve all the rust fast enough.
The electrolytic solution will have to work harder to keep up with the rust removal. As a result, it will take longer to accomplish the task.
Conversely, if the anode has a wide surface area, then the amperage output will be maximized and therefore will have enough current density to dissolve all the rust fast enough.
The efficiency of electrolysis depends on a couple of factors such as the amount of current and the size of the electrodes. In order to maximize the efficiency of the process, we need to control the current density.
In other words, the smaller the distance between the anode and cathode, the more concentrated the current becomes.
It has also been discovered that the heavier the object you're de-rusting and the lighter the sacrificial metals are, the faster the rate of electrolysis.
Does Increasing The Voltage Hasten the Electrolysis Process?
Increasing the voltage does not necessarily mean an increase in amperage. An increase in voltage will only produce excess heat and will not increase the efficiency of your electrolysis unless you have an increase in amperage.
A low-grade power supply and a high resistance will always produce very little current even at high voltage.
What Car Battery Should I Use For Electrolysis?
When choosing a battery source to be used for electrolysis, the voltage should be the first consideration. You need to have a 24-volt-DC car battery for this process to work.
Voltage affects the amount of electricity that will be passed through the cell to generate the desired current for the electrolytic bath.
Does Temperature Affect the Electrolysis Process?

One major problem that may arise in the electrolysis process is that the amount of hydrogen obtained is relatively low. The reason for this is that the electrical current output is usually lower than what the system is capable of producing.
To obtain a higher yield of hydrogen, you must increase the amperage. In this case, the current is supplied through a direct current (DC) battery rather than the alternating current (AC) source.
This requires the proper application of DC voltage to the cathode electrode and the proper configuration of the circuit.
Hydrogen is produced by electrolysis through the electrolytic decomposition of water and one of the requirements for the process to be fast and efficient is to have a high temperature. The rate of water decomposition is directly proportional to the temperature of the solution.
As the temperature increases, the speed at which the water molecules split increases. In other words, the reaction rate is amplified with every degree of Celsius.
In order to get the best performance of the electrolysis process, the electrolytes must be kept at a temperature above 40 °C and below 100 °C. These figures may vary depending on the metal you're working on but generally, this is where you'll want your temperature to be.
How Do You Increase The Rate of Efficiency of Electrolysis?

To increase the efficiency of the electrolysis process, you must take into account the properties of the electrolytes.
You can increase the efficiency of electrolysis by using salt and baking soda. These substances can facilitate the process by increasing the electrolytes in the solution.
A mixture of salt and baking soda in a gallon of water with a ratio of 25:1 produces the best result. The unit of measurement for salt and baking soda is in tablespoons.
Can I Use Epsom Salt For Electrolysis?
Epsom salt will enhance the effectiveness of an electrolysis system, which will improve the quality of your output and reduce the duration of the treatment.
This is because Epsom salt is a gold mine of electrolytes that help carry and maximize the efficiency of the electrical current in the ionization of water molecules.
Check out this Epsom salt on Amazon.
Can I Use Distilled Water in Electrolysis?
Distilled water is not commonly used to make battery electrolyte solutions because of the fact that it's distilled. It doesn't contain salts or minerals that will facilitate the chemical reaction required for electrolysis.
Tap water is much more effective because it's easily accessible and it contains some salt.
What Materials Can Be Best Used as Anodes?
Anodes are a critical component to the success of an electrolysis process. Materials that can be used for anodes are iron, carbon steel, zinc, and lithium.
The common anode material is usually iron, though other metals that are not corrosion-resistant have been utilized, depending on availability.
Is It Okay to Clean and Reuse an Anode Rod?

An anode rod is used to remove rust in the electrolytic solution. It is used as part of the corrosion migration process from the cathode.
An anode can be reused if that's what you want. However, after the electrolysis is completed, you'll notice that the anode becomes too corroded that you wouldn't even think of cleaning and reusing it for another cycle.
This is why it's recommended to use an iron or steel bar that serves no utility anymore to be used as an anode.
In Closing
Electrolysis is a technique where you use a DC power source to split the water into positively and negatively charged water molecules through ionization. A chemical reaction occurs when the hydrogen molecules and oxygen molecules bond with the electrodes.
To perform electrolysis, you will need a DC power supply. A typical car battery charger is suitable for this task. An electrolytic solution needs the proper amount of voltage to function properly.
You might also like:




I write this 5-22-23 to ask: Where do you purchase a “true” manual battery charger? I have used Electrolysis to clean cast iron for 15 years. I went to market to buy a new charger and cannot find one. Some are marked Manual but do not work for the process. Any suggestions?